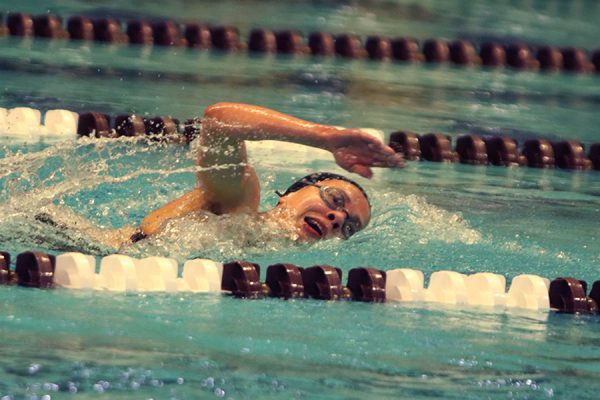A Mole Kingdom
A New Chemistry Project Emerges
Photo courtesy of Aahana Mulchandani
Waiting to be graded, the sewn fabric moles sit on top of the lab table. These mole projects revolve around teaching students more about moles with a creative approach. “I wanted them to learn something about perhaps an element they aren’t as familiar with,” Cieri said. “I also wanted them to understand how all the elements have different masses.”
October 29, 2021
English teachers use essays, math teachers use homework and chemistry teacher Christopher Cieri uses mole projects to teach students. Moles, not the animal, are a scientific unit of measurement used to measure huge amounts of small items such as atoms, molecules or particles.
Students in chemistry were given two options for their projects: make a mole and write a paragraph about their chosen element or calculate the mole of an item of their choice. For the stuffed mole project, students were given a mole template that they would use to construct and sew their mole. They were allowed to choose an element on the periodic table that they would represent in mole form.
However, the students had to make sure that their mole had the same molar mass as the element they chose. The calculation aspect of the mole was more about a student demonstrating how large a mole of any common object could be by showing their work and creating a colorful project on paper.
According to Cieri, he wanted the students to have fun with their projects while learning more about molar mass and weight and expressing creativity. He said he also wanted students to learn more about molar mass as well as how large a mole really is.
“I wanted them to learn something about perhaps an element they aren’t as familiar with,” Cieri said. “I also wanted them to understand how all the elements have different masses.”
According to sophomore Thomas Dow, a student in Advanced Chemistry, his creative thinking was a huge factor in his project. He said it was a matter of creativity and originality with his cesium stuffed mole.
“I chose Cesium because I think it is an original metal,” Dow said. “Not a lot of people would think of it when they think of moles; they think of potassium, iron and gold.”
Dow decided to create the stuffed mole. It took him one hour and thirty minutes to create it.
“I would still choose the mole project, because calculations, to me, don’t show any creativity,” Dow said. “You have to introduce creativity when creativity is a bonus. [In] this one, creativity is quantifiable.”
Sophomore Amanda Ce used Titanium as her element, and she constructed a mole that looked like the singer, Sia.
“I had always thought it was a cool metal and it had first reminded me of Sia’s song ‘Titanium,'” Ce said. “I made my mole to look like Sia with her signature black and white bangs covering her eyes, pursed lips and her large black bow at the top of her head.”
Not only that, Ce also said she learned a lot about her metal during the project.
“The most important thing about making this project is finding out what the element chosen can really be used for,” Ce said. “It’s not just making the mole; I also had to research and type a paragraph about what titanium is used for, and the information surprised me, I found that it is used for many items we use everyday.”
On the other hand, students such as sophomore James Sanderson, used calculations to learn more about this scientific unit of measurement. Sanderson calculated how many donuts are needed to wrap around the circumference of Saturn for it to make up a mole.
“It only took me a night, but I did have to spend a few hours on the calculation alone,” Sanderson said. “I can’t sew, which is why I decided to go for the mole conversions.”
According to Sanderson, the sewing aspect of moles can be tricky and the complexity of the stuffed mole was overwhelming.
“It seems a bit more challenging to see all requirements be met for that version of the project, since for the calculation I really just had to do some conversions and make the paper look neat and colorful,” Sanderson said. “For the mole, you needed to do plenty more, which is part of the reason I didn’t do that version.”
Sophomore Arav Neroth also chose the mole calculations over the mole project because he cannot sew.
“I calculated how many candles could fill how many chandeliers to fill the surface of Saturn,” Neroth said. “I chose the project because of my friend, and we thought about how that much wax could suffocate the entire world. It was an original project because no one else would think of chandeliers which is what I was thinking of doing.”
Cieri said he thought that sewing a mole would be another way to help students with the “Wow!” factor and give the students a sense of pride.
“Sewing is a life skill, so it’s kind of cool to be like ‘Oh I sewed this thing together,’” Cieri said. “I don’t think it’s easy, especially if it’s a well-made one.”
Cieri said he wanted the students to express what they learned the past six weeks during class while they worked through the mole unit.
“I also wanted them a chance to show some creativity,” Cieri said. “Whether [a student] did the stuffed mole or the calculation, it was an opportunity for them to show creativity.”
For Ce, she said she was able to harness her creativity as well as have fun with her project.
“I felt very accomplished and well educated after I finished the project,” Ce said. “I loved how my mole looked and I learned a lot about titanium and its uses.”
Neroth said he had an unexpected takeaway from his unusual project choice.
“My entire goal was to be original,” Neroth said. “No one would think of wax, chandeliers and Saturn to try to make a project. So in the end, I was able to accomplish my goal and I had a lot more fun than I thought I would.”
Students learned lessons from the projects, including the concept of the mole as a unit of measurement. Whether it was sewing a mole or calculating a mole to show its size, students were able to show what they learned about their subject in a creative way.
“From the projects I definitely took away a better understanding of the sheer size of a mole,” Sanderson said. “I learned about how something larger than a minuscule molecule can represent itself in gargantuan proportions when we imagine a full mole of the object.”

![Posing with their UIL State Trophy, the Robolobos Van Halen Team beams with excitement after their win. “It was a team effort,” junior Noah Vo said. “I was happy because something happened in the first match and the match was also really close. So [when] they finally revealed it, I was pretty happy.” Photo courtesy of Amy Lovelace](https://cphswolfpack.com/wp-content/uploads/2025/05/IMG_0910-EDIT-1200x723.jpg)

![Broadcast, yearbook and newspaper combined for 66 Interscholastic League Press Conference awards this year. Yearbook won 43, newspaper won 14 and broadcast took home nine. “I think [the ILPC awards] are a great way to give the kids some acknowledgement for all of their hard work,” newspaper and yearbook adviser Paige Hert said. “They typically spend the year covering everyone else’s big moments, so it’s really cool for them to be celebrated so many times and in so many different ways.”](https://cphswolfpack.com/wp-content/uploads/2025/05/edited-ILPC.jpg)













![Bringing her arm over her head and taking a quick breath, junior Lauren Lucas swims the final laps of the 500 freestyle at the regionals swimming competition on date. Lucas broke the school’s 18-year-old record for the 500 freestyle at regionals and again at state with a time of 4:58.63. “I’d had my eye on that 500 record since my freshman year, so I was really excited to see if I could get it at regionals or districts,” Lucas said. “ State is always a really fun experience and medaling for the first time was really great. It was a very very tight race, [so] I was a bit surprised [that I medaled]. [There were] a lot of fast girls at the meet in general, [and] it was like a dogfight back and forth, back and forth.” Photo by Kaydence Wilkinson](https://cphswolfpack.com/wp-content/uploads/2025/03/Kaydence-2.7-23-edit-2.jpg)
![As the support team sits and poses for a photo in the cafeteria with the counseling team they eagerly wait to start their day. "We [all] seem to be a team, I get up every day and there's days where I don't want to go to work today, but I'm thankful that I have a job and I'm blessed to have what I have," Christopherson said. Photo Courtesy of Julie Weltens.](https://cphswolfpack.com/wp-content/uploads/2025/01/AF9E8470-10D7-4C91-BF28-EC8F86BAB66C-1200x852.jpeg)
![Officer Stephanie Cash is in her second year as an SRO at CPHS. “Seeing [students] grow over the years has been kind of cool,” Officer Cash said. “Freshmen that [are] all over the place and then in the next couple of years get a little more squared away and go to class and do work and start thinking about the future. Being a part of a student's growth is the best way to measure my success as an SRO.” Photo Courtesy of Cedar Park Police Department's PIO, Alicia Gallagher.](https://cphswolfpack.com/wp-content/uploads/2024/12/CPHS-SRO-900x1200.jpg)



![Taking a breath as he raises his arm up and out of the water, sophomore Kaden Padilla swims the 500 freestyle at the UIL state meet on Feb. 21-22. Padilla placed 10th overall and second in the consolation final in the event, dropping two seconds. “My family was there, so being able to drop time for them was really special,” Padilla said. “It was awesome [finding out I advanced to the consolation finals]. I wasn’t expecting it, and I was very surprised. My parents being there definitely made me a lot happier knowing they got to see me swim in finals.” Photo by Skyler King.](https://cphswolfpack.com/wp-content/uploads/2025/03/kaden-padilla.jpg)

![Three defenders try to stop senior point guard Hope Edwards before the ball leaves her hands. The girls basketball team faced Liberty Hill on Feb 21, losing 58-40. “[My season was] definitely bittersweet,” Edwards said. It's definitely sad [because] I'm gonna miss all my teammates, my coaches and just the whole CP environment.”](https://cphswolfpack.com/wp-content/uploads/2025/03/julia-128-1200x800.jpg)